Abstract
Background: HM43239 is a novel FMS-like tyrosine kinase 3 (FLT3) inhibitor with potent in vitro and in vivo activity against both FLT3 mutated and wild-type AML. Preclinically HM43239 was highly active against FLT3 internal tandem duplication (ITD) mutated as well as resistance-conferring D835 and gatekeeper (F691) tyrosine kinase domain (TKD) mutated AML cell lines and xenograft models (AACR2021 abstract 1257). In addition to FLT3, HM43239 inhibits phosphorylation of spleen tyrosine kinase (SYK) known to be highly activated in AML and associated with resistance to FLT3 targeted therapy. We report data from an ongoing Phase 1/2 dose escalation and expansion FIH study assessing the safety, efficacy, pharmacokinetics (PK) and pharmacodynamics (PD) of HM43239 in pts with R/R AML.
Methods: A phase 1/2 trial was conducted to evaluate HM43239 in pts with R/R AML who had received at least 1 prior line of therapy, including ≥ 1 prior FLT3 inhibitors (FLT3i). Pts with or without a FLT3 mutation (FLT3m) received HM43239 orally once daily until unacceptable toxicity or no clinical benefit (each cycle of ~28 days). Doses 20 to 160 mg had been evaluated at the time of this abstract. The dose escalation was initiated with an accelerated titration design followed by a 3+3 design. The 3+3 design kicked in after the first instance of a dose limiting toxicity (DLT) or moderate toxicity (MT, a grade 2 non-hematologic adverse event (AE) judged by the investigator to be at least possibly related to study drug) occurred. Parallel backfill expansion of cohorts deemed safe were initiated based on the observed safety and efficacy in the dose-escalation. AEs were graded per NCI-CTCAE (v4.03). Responses were evaluable according to the international working group (IWG) AML criteria including complete remission (CR) and composite CR (CRc). PK was examined after single and multiple dose (15 or 17 days) of 20 to 160 mg. Inhibition of FLT3 phosphorylation (pFLT3) was evaluated by a plasma inhibitory activity (PIA) assay and correlated with exposure levels.
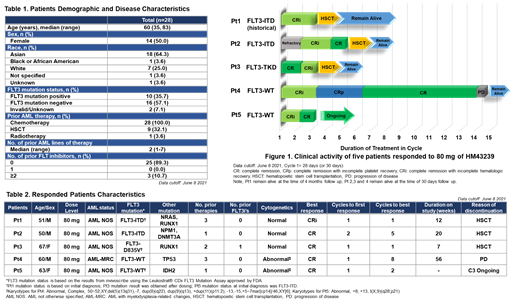
Results: Twenty-eight pts were enrolled at multiple international centers between March 2019 and June 2021: 13 in the dose escalation cohorts 20 - 160 mg and 15 in the dose expansion cohort at 80 mg. Patient characteristics are shown in Table 1. Median number of prior therapies for AML in this R/R pts was 2 (range, 1-7). Ten pts (36%) had FLT3m, 16 pts (57%) had FLT3 wild-type (FLTwt), and 2 pts (7%) were FLT3 unknown at enrollment. Most frequently reported FLT3m was ITD (21%) followed by TKD (11%), and ITD/TKD (4%). After 1 pt in the 80 mg dose escalation cohort showed a MT (Grade 3 nausea), the accelerated titration design was switched to the 3+3 design for 80 mg and higher doses. The most common drug related treatment-emergent adverse events (TEAEs) were diarrhea (14%), nausea (7%), vomiting (7%) and alanine aminotransferase increased (7%), and no drug related > Grade 3 TEAEs were observed to date. Five of 28 pts treated at all dose levels achieved CRc (17.9%) (Table 2). Best response was IWG CR in 4 pts and IWG CRi in 1 pt. All 5 CRc pts were treated at the 80 mg dose (n= 19) resulting in CRc of 26.3% at the 80 mg dose. Of 8 FLT3m pts at 80 mg, CRc was achieved in 3 pts (37.5%) and one had received prior FLT3i, gilteritinib. Of 11 FLT3wt pts at 80 mg, CRc was achieved in 2 pts (18%), including a relapsed TP53m AML who achieved CR and remained on study for 12 months before progressing. Among the remaining 4 CRc pts, three proceeded to hematopoietic stem cell transplantation (HSCT) remaining alive and in remission post HSCT at this time, and one remains in CR in Cycle 3 (Fig 1). The plasma concentration showed dose-dependent increase after single administration of 20 to 160 mg. After 17 days of treatment, steady-state was reached and PIA assay showed dose-dependent inhibition of pFLT3 with up to 90% at the dose levels ≥ 80 mg.
Conclusions: HM43239 a preclinically potent FLT3 and SYK inhibitor showed a favorable safety profile with only mild AEs and no DLTs in this ongoing Phase 1/2 study. At 80 mg dose, HM43239 demonstrates clinical activity in both FLT3m (including a prior gilteritinib failure pt) and FLT3wt AML (including >1 year CR without HSCT in a relapsed TP53m AML). We continue to further evaluate doses above 80 mg to determine the optimal recommended Phase 2 Dose (RP2D). The dose escalation cohort of 160 mg and the dose expansion cohort of 120 mg are currently enrolling and updated response, safety, and PK/PD data will be presented. NCT03850574.
Daver: Pfizer: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Trovagene: Consultancy, Research Funding; Hanmi: Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; Trillium: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Lee: Ingenium Therapeutics, Daejeon, Korea: Consultancy, Current holder of individual stocks in a privately-held company. Arellano: Syndax Pharmaceuticals, Inc: Consultancy; KITE Pharma, Inc: Consultancy. Yoon: Hanmi Pharmaceutical: Current Employment. Lee: Hanmi Pharmaceutical: Current Employment. Kim: Hanmi Pharmaceutical: Current Employment. Lee: Hanmi Pharmaceutical: Current Employment. Jonas: AbbVie, BMS, Genentech, GlycoMimetics, Jazz, Pfizer, Takeda, Treadwell: Consultancy; 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo Oncology, Pfizer, Pharmacyclics, Sigma Tau, Treadwell: Research Funding; AbbVie: Other: Travel reimbursement. Baek: Hanmi Pharmaceutical: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal